The fuel cell is an electrochemical devise, which converts chemical energy
of the fuel to electricity by combining gaseous hydrogen with air in the absence
of combustion. The basic principles of operation of the fuel cell is similar to
that of the electrolyser in that the fuel cell is constructed with two
electrodes with a conducted electrolyte between them. 
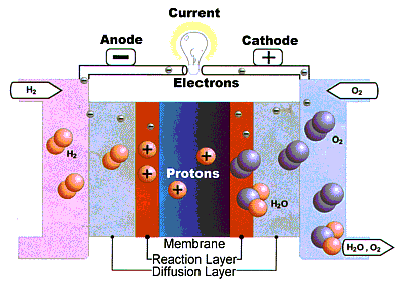
For a better explanation of the fuel cell operation, take a closer look at the Proton Exchange Membrane (PEM) fuel cell (click on the picture for animation)
The heart of the cell is the the proton conducting solid PEM. It is surrounded by two layers, a diffusion and a reaction layer. Under constant supply of hydrogen and oxygen the hydrogen diffuses through the anode and the diffusion layer up to the platinum catalyst, the reaction layer. The reason for the diffusion current is the tendency of hydrogen oxygen reaction.
Two main electrochemical reactions occur in the fuel cell. One at the anode (anodic reaction) and one at the cathode.
At the anode, the reaction releases hydrogen ions and electrons whose transport is crucial to energy production.
H2®2H+ + 2e-
The hydrogen ion on its way to the cathode passes through the polymer membrane while the only possible way for the electrons is though an outer circuit. The hydrogen ions together with the electrons of the outer electric circuit and the oxygen which has diffused through the porous cathode reacts to water.
2H+ + ½ O2 + 2e- ®H2O
The water resulting from this reaction is extracted from the system by the excess air flow. The reaction is:
H + ½ O2
®H2O
This process occurs in all types of fuel cells.
